Gigantactinidae
Whipnose Seadevils
Theodore W. Pietsch

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
The Gigantactinidae is one of the most well-defined and highly specialized families of deep-sea anglerfishes. The females are readily distinguished from those of the other ten families of the suborder by having an elongate streamlined shape, a relatively small head and slender caudal peduncle, five pectoral radials, and a greatly prolonged illicium that reaches a length between one and four times standard length. The family includes 22 species in two genera.
As is the case with nearly all ceratioid groups, little is known about the ecology of the gigantactinids. However, recent evidence suggests that this group lives a benthic lifestyle and may even swim upside down while foraging, drifting motionless as the esca lures prey off the bottom (Moore, 2002). ROV Jason, of Woods Hole Oceanographic Insitutue, captured a whipnose anglerfish (Gigantactis sp.) on video (Hawaii-2 Observatory video clips) swimming (see clip fish_gig_1_1) and apparently foraging upside down (see clip fish_gig_1_3).
Characteristics
Diagnosis
Metamorphosed females of the Gigantactinidae differ from those of all other ceratioid families in lacking the vomer, mesopterygoid, and an ossified scapula; in having the preopercle reduced to a short narrow strut of bone; the interopercle reduced, without ligamentous connection to the angular; the caudal fin emarginate (except in the largest known females of Gigantactis kreffti and G. macronema), with nine caudal-fin rays, the ventralmost ray reduced and embedded within skin surrounding the adjacent ray; the pterygiophore of the illicium exceptionally large, the compressed posterior end butting up against the supraoccipital; and five pectoral radials (Bertelsen et al., 1981).
Metamorphosed females are further distinguished by having the following combination of character states: supraethmoid greatly reduced, usually absent (a tiny rudiment observed in a single specimen; Bertelsen et al., 1981:10, fig. 12); frontals narrow, widely separated, and without ventromedial extensions in Gigantactis, absent in Rhynchactis; parietals greatly reduced in Gigantactis, absent in Rhynchactis; sphenotic spines absent; pterosphenoid absent; metapterygoid present; hyomandibular with a single head; hypohyals 1 in Gigantactis, 2 in Rhynchactis; branchiostegal rays 6 (2 + 4), very rarely 7 (2 + 5); opercle reduced, deeply bifurcate posteriorly to form two slender forks, ventral fork slightly longer than dorsal fork; subopercle long and slender, ventral part in Gigantactis becoming more reduced with age and splitting to form a small projection on anterior margin and as many as three filamentous, posteriorly directed prolongations; quadrate, articular, angular, and preopercular spines absent; jaws subequal, upper jaw extending anteriorly beyond lower jaw; dentaries without posterior bifurcation and without symphysial spine, attached to each other at symphysis by thick elastic connective tissue; premaxillae without ascending or postmaxillary processes, long and narrow, well ossified in Gigantactis, greatly reduced in Rhynchactis; maxillae reduced to threadlike ossification or absent; anterior-maxillomandibular ligament absent; pharyngobranchials I and IV absent; pharyngobranchials II and III extremely large, well ossified, and heavily toothed; epibranchial I absent; anterior half of epibranchials III and IV fused; ceratobranchial I reduced, represented only by short posterior portion and tiny isolated remnants of anterior portion; ceratobranchial V absent except for tiny isolated remnants; ossified hypobranchials and basibranchials absent; epibranchial and ceratobranchial teeth absent; remnants of ceratobranchial I embedded in tissue of lateral wall of pharynx; proximal one-quarter to one-half of ceratobranchials II-IV closely bound together by connective tissue, displacing gill filaments; epurals absent; hypural plate entire, without posterior notch; pterygiophore of illicium bearing a small ossified remnant of second cephalic spine; escal bulb and central lumen present in Gigantactis, absent in Rhynchactis; esca of Gigantactis without large tooth-like denticles; coracoid reduced in Gigantactis (with an elongate cartilaginous posteroventral process), unossified in Rhynchactis (posteroventral process absent); pelvic bones absent in Gigantactis, short cylindrical remnants in Rhynchactis; dorsal-fin rays 3-9; anal-fin rays 3-7; pectoral-fin rays 14-22; caudal-fin rays 9 (ventralmost ray extremely short, covered with skin); females of Gigantactis with all caudal-fin rays simple, Rhynchactis and males of Gigantactis with 2 simple+ 4 bifurcated + 3 simple; numerous close-set dermal spinules covering entire head, body, and fins of Gigantactis, extending out onto illicium and in some species onto esca (visually obvious in stained specimens of females of all species without microscopic aid); minute dermal spinules present in skin of largest known females of Rhynchactis, but absent in smaller specimens; ovaries paired; pyloric caeca absent.


Free-living male of Gigantactis male group I, 14.5mm, ZMUC P921533 (after Bertelsen et al., 1981). © 1981, E. Bertelsen et al., K. Elsman
Metamorphosed gigantactinid males differ from those of all other ceratioid families in having the following combination of character states: eyes minute, only 3-5% SL in most specimens; olfactory organs large, depth 8-10% SL in most specimens; anterior nostrils close together, directed anteriorly; premaxillae degenerate, but maxillae well developed; jaw teeth absent; denticular teeth all or nearly all mutually free; upper denticular teeth 3-6 (rarely 2), not connected to pterygiophore of illicium; lower denticular teeth 4-7 (rarely 3); hyomandibular with a single head; branchiostegal rays 6 (rarely 7); pectoral radials 5; fin-ray counts as given for metamorphosed females; pelvic bones absent; skin naked or densely covered with dermal spinules; free-living, no evidence that males ever become parasitic, but temporarily attached males are unknown (see Pietsch, 2005).
In addition to the sexual dimorphism common to all metamorphosed ceratioids, gigantactinid males differ further from females of the family in having a symphysial cartilage, a vomer, and a basibranchial ossification; they differ also in having fully developed frontals, parietals, opercular bones, and ceratobranchials (Bertelsen et al., 1981, figs. 11D-F, 14, 15).
Gigantactinid larvae differ from those of all other ceratioid families in having exceptionally large pectoral fins (length 45-55% SL), comparable only to those of the Caulophrynidae; they differ further in having the following combination of character states: short, nearly spherical; skin highly inflated; pelvic fins absent; fin-ray counts as given for metamorphosed females; sexual dimorphism evident, females with a small, club-shaped illicial rudiment protruding from head; metamorphosis beginning at 8-10 mm SL, metamorphosal stages 9-20 mm SL (Bertelsen, 1981, 62, 1984:326, fig. 168A-B).
Description
Metamorphosed females with body slender, streamlined; length of head (measured from the tip of the snout to the base of the pectoral fin) about 25% SL; greatest depth of body about 25% SL; length of caudal peduncle 20-30% SL, depth of caudal peduncle 5-10% SL; caudal fin deeply cleft in most species, some with one or two dorsal- and ventralmost rays greatly prolonged; snout of Gigantactis pointed, protruding well beyond anterior margin of jaws, more blunt in Rhynchactis; mouth large, opening horizontal, cleft extending past eye; oval valves absent; olfactory organs raised on short cylindrical stalks; dentition highly variable: Gigantactis with premaxillary teeth small, depressible, relatively few, arranged in one or two series; dentary teeth larger, depressible, more numerous, arranged in 2-6, more-or-less distinct overlapping series, external series situated on outer margin of jaw; several anteriormost and lateralmost dentary teeth greatly enlarged into curved fangs; metamorphosed females of Rhynchactis with jaws toothless (except for a few premaxillary teeth retained in juveniles); in both genera vomerine teeth absent, upper pharyngeal teeth extremely large, lying in a forward position in roof of mouth; epibranchial I absent; epibranchials II-IV closely bound together; remnant of ceratobranchial I free from wall of pharynx; proximal one-quarter to one-third of ceratobranchial III bound to ceratobranchial IV; epibranchial IV and ceratobranchial IV bound to wall of pharynx, no opening behind fourth arch; gill filaments present on epibranchials II-IV and full length of ceratobranchials II-IV; discrete pseudobranch absent, but at least some species with a few gill filaments on wall of pharynx adjacent to epibranchial II; pterygiophore of illicium shorter than skull, nearly immobile, its short protruding anterior end forming tip of snout of Gigantactis, completely concealed under skin of head in Rhynchactis; illicium greatly prolonged, length highly variable among species (shortest about 40-100% SL in G. longicirra, longest 340-490% SL in G. macronema), reaching full relative length at a standard length of about 30 mm; Gigantactis with an elongate, club- or spindle-shaped escal bulb containing a relatively small, internal, spherical photophore, and bearing appendages in form of papillae, filaments, and lobes; filaments on stem of illicium present or absent; Rhynchactis without escal photophore, bioluminescent bacteria absent (in sharp contrast to all other ceratioid females except those of Caulophrynidae and Neoceratiidae); illicium of some specimens bearing elongate slender filaments of complex structure and unknown function; organs of acoustico-lateralis system on short stalks of pigmented skin, more or less distinctly connected in series by narrow unpigmented grooves, pattern of placement as described for other ceratioids (see Bertelsen et al., 1981:8, 10, fig. 8).
Color of females in preservative uniformly dark brown to black over entire external surface of head, body, and fins (except for unpigmented transparent parts of escal bulb, escal appendages, and stem of illicium; for ontogenetic and specific variation, see species accounts below); oral cavity more or less pigmented. Skin of metamorphosed males weakly pigmented in Rhynchactis, varying among species-groups of Gigantactis from unpigmented to dark brown or black. Subdermal pigment of larvae, males, and juvenile females varying between genera and among species groups of Gigantactis from absent (e.g., Gigantactis longicirra) to dense, dorsal and peritoneal groups of melanophores (e.g., Rhynchactis).
Size and Sexual Maturity
Gigantactinids are among the largest known ceratioids. While some of the 30 or so females of Gigantactis greater than 200 mm have relatively large ovaries, none contain ripe or ripening eggs. Eggs larger than about 0.5 mm in diameter have not been found. At the same time, none of the more than 175 metamorphosed females in collections around the world are parasitized by males, thus it is assumed that males remain free-living. The largest known male, a member of the Gigantactis Male Group I measures 22 mm and has ripe testes.
Discussion of Phylogenetic Relationships
Rhynchactis has undergone such a drastic reduction and loss of parts that clearly it is the more derived of the two gigantactinid genera. Within the genus Gigantactis, there are four morphological trends that seem to characterize anglerfish evolution in general (Pietsch, 1972, 1974:87, 88): (1) an increase in the length of the illicium, (2) a decrease in the number of median-fin rays, (3) a loss of jaw teeth, and (4) an increase in morphological complexity of the luring apparatus, in this case, reflected in a general tendency to increase the number of distal filaments of the esca and filaments of the illicium. Giagantactis longicirra appears to be the least derived member of the genus, having the shortest illicium, the greatest number of longitudinal series of dentary teeth, the highest dorsal-ray count, and the least number of distal escal filaments. Members of the G. macronema group (G. macronema, G. microdontis, and G. ios) are the most derived, having the longest illicium, the fewest series and total number of dentary teeth, the lowest dorsal-ray counts, and numbers distal, escal filaments. The remaining species of the genus are more or less intermediate in specialization. Members of the G. vanhoeffeni group, include G. vanhoeffeni, G. meadi, G. gibbsi, G. gracilicauda, and G. paxtoni, are united in sharing a relatively short illicium and a similar escal morphology. Members of the G. gargantua group, including G. gargantua, G. watermani, and G. herwigi, likewise share a similar escal morphology but are also united on the basis of having a relatively long illicium and an elongation of the second and seventh caudal-fin rays.
Distribution
Both genera of the family are represented in all three major oceans of the world. Females of Gigantactis have been reported from as far north as southern Greenland, with the southernmost record close to 50°S in the Atlantic sector of the Southern Ocean. The known distribution of Rhynchactis is restricted to tropical and subtropical regions between about 30°N and 10°S. The great majority of the recorded metamorphosed specimens of the family have been captured in nets fished in maximum depths exceeding 1000 m. Gigantactinids thus appear to be among the deepest-living ceratioids, with greatest abundance between 1000 and at least 2500 m.
Key to the Genera of the Gigantactinidae
Females
1A. Teeth of lower jaw well developed, in several rows; dorsal-fin rays 5-9, rarely 4 or 10; anal-fin rays 4-7, rarely 8; escal bulb present (Gigantactis Brauer, 1902)
1B. Lower jaw teeth absent; dorsal-fin rays 3-4, rarely 5; anal-fin rays 3-4; escal bulb absent (Rhynchactis Regan, 1925)
Males
1A. Upper denticular teeth 3, lower denticular teeth 4; dorsal-fin rays 5-9, rarely 4 or 10; anal-fin rays 4-7, rarely 8; skin spinulose in some species (Gigantactis Brauer, 1902)
1B. Upper denticular teeth 4, lower denticular teeth 6; dorsal-fin rays 3-4, rarely 5; anal-fin rays 3-4; skin naked (Rhynchactis Regan, 1925)
References
Bertelsen, E. 1951. The ceratioid fishes. Ontogeny, taxonomy, distribution and biology. Dana Rept., 39, 276 pp.
Bertelsen, E., T. W. Pietsch, and R. J. Lavenberg. 1981. Ceratioid anglerfishes of the family Gigantactinidae: Morphology, systematics, and distribution. Nat. Hist. Mus. L. A. Co., Contri. Sci., 332, vi , 74 pp.
Bertelsen, E., and T. W. Pietsch. 1998. Revision of the deepsea anglerfish genus Rhynchactis Regan (Lophiiformes: Gigantactinidae), with descriptions of two new species. Copeia, 1998(3): 583-590.
Brauer, A. 1902. Diagnosen von neuen Tiefseefischen, welche von der Valdivia-Expedition gesammelt sind. Zool. Anz., 25, 668(4): 277–298.
Moore, J.A. 2002. Upside-Down Swimming Behaviour in a Whipnose Anglerfish (Teleost: Ceratioidei: Gigantactinidae). Copeia, 2002(4): 1144–1146.
Pietsch, T. W. 1972. Ergebnisse der Forschungsreisen des FFS "Walther Herwig" nach Südamerika. XIX. Systematics and distribution of ceratioid fishes of the genus Dolopichthys (family Oneirodidae), with the description of a new species. Arch. FischWiss., 23(1): 1–28.
Pietsch, T. W. 1974. Osteology and relationships of ceratioid anglerfishes of the family Oneirodidae, with a review of the genus Oneirodes Lütken. Nat. Hist. Mus. L. A. Co., Sci. Bull., 18, 113 pp.
Pietsch, T. W. 2005. Dimorphism, parasitism, and sex revisited: modes of reproduction among deep-sea ceratioid anglerfishes (Teleostei: Lophiiformes). Ichthyol. Res., 52: 207–236.
Regan, C. T. 1912. The classification of the teleostean fishes of the order Pediculati. Ann. Mag. Nat. Hist., Ser. 8, 9(28): 277–289.
Regan, C. T. 1925. New ceratioid fishes from the N. Atlantic, the Caribbean Sea, and the Gulf of Panama, collected by the "Dana." Ann. Mag. Nat. Hist., Ser. 8, 8(62): 561–567.
Title Illustrations

| Scientific Name | Gigantactis |
|---|---|
| Specimen Condition | Dead Specimen |
| Sex | Female |
| Image Use |
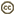 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© Theodore W. Pietsch

|
About This Page
Theodore W. Pietsch

University of Washington, Seattle, Washington, USA
Correspondence regarding this page should be directed to Theodore W. Pietsch at and Christopher P. Kenaley at
Page copyright © 2005 Theodore W. Pietsch
 Page: Tree of Life
Gigantactinidae. Whipnose Seadevils.
Authored by
Theodore W. Pietsch.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Gigantactinidae. Whipnose Seadevils.
Authored by
Theodore W. Pietsch.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 06 November 2005
Citing this page:
Pietsch, Theodore W. 2005. Gigantactinidae. Whipnose Seadevils. Version 06 November 2005 (under construction). http://tolweb.org/Gigantactinidae/22011/2005.11.06 in The Tree of Life Web Project, http://tolweb.org/








 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site