Incirrata
Common octopods, octopuses or devilfishes
Katharina M. Mangold (1922-2003) and Richard E. Young- Argonautoidea Naef, 1912
- Octopodoidea Orbigny, 1839
- Paleoctopodidae

Introduction
Comments on this page refer to living Incirrata.
Incirrate octopods are about 85% of all octopodan species. They are very small to large in size. The members of the Octopodidae, the family with the most species and individuals, are benthic. The other seven incirrate families have pelagic species. The benthic incirrate octopods live from the intertidal zone to at least 4000 m, and bathypelagic forms may be found down to 2000 m. The octopodids exhibit sophisticated behavior. The brain of benthic octopuses, especially that of Octopus vulgaris, has become a model for relating brain structure to function (e.g., J. Z. Young, 1971). Incirrate octopods are commercially fished throughout the world.
Fins are absent and the arms bear one or two rows of suckers and lack cirri. The shape of the body is saccular and rather broad. The arms are often of about equal length, but sometimes the dorsal or lateral or ventral arms are distinctly longer than the others.
Brief diagnosis:
An octopod ...
- without fins.
- without cirri on arms.
Characteristics
- Arms
- One member of Arms III hectocotylized.
- Cirri absent from arms.
- Arms without internal horizontal septa (except Japetella).
- Suckers uniserial or biserial.
- Head
- Beaks: For an introduction to pelagic incirrate beaks go here.
- Eyes: Cornea present (greatly reduced in some pelagic species).
- Beaks: For an introduction to pelagic incirrate beaks go here.
- Fins
- Absent.
- Absent.
- Gills
- Gills with branchial canals.
- Gills asymmetrical in cross-section.
- Viscera
- Paired oviducts present.
- Egg chorion with stalk.
- Typical spermatophores present.
- Posterior salivary glands located posterior to cephalic cartilage.
- Radula and ink sac usually present.
- Shell
- Shell a pair of stylets or absent.
Nomenclature
Naef (1923) placed the argonautoid families within a single family, the Argonautidae. Robson (1932) placed these families in the Tribe Argonautida and the remaining families in the Tribes Heteroglossa and Ctenoglossa based on the structure of the radula. The latter tribe contained the families Bolitaenidae and Amphitretidae. However the placement of these families in their own clade has been questioned (e.g., Thore, 1949; Voight, 1997). Strugnell et al., (2013) divided the Incirrata into two superfamilies, the Argonautoidea containing four families and the Octopodoidea containing six families one of which, the Amphitretidae, combined three former families, Amphitretidae, Bolitaenidae and Vitreledonellidae.Idioctopus gracilipes described by Taki (1962, 1964) and placed in its own family is probably a synonym of Amphitretus pelagicus (see Hochberg, et al, 1992).
Discussion of Phylogenetic Relationships
by Jan Strugnell*
Strugnell et al., (2013), analysed three nuclear (rhodopsin, octopine dehydrogenase, pax-6) and four mitochondrial (12S rRNA, 16S rRNA, COI, COIII) genes from 36 coleoid taxa. This comprised nine outgroup taxa, representing the five major lineages of the Decapodiformes (Oegopsida, Sepiolida, Myopsida, Spirulida and Sepiida). The Octopodiformes was represented by Vampyromorpha (Vampyroteuthis infernalis), Cirrata (Opisthoteuthis massyae and Stauroteuthis gilchristi) and 27 species belonging to the Incirrata (including representatives of the five previously proposed subfamilies [Eledoninae, Octopodinae, Bathypolypodinae, Megaleledoninae and Graneledoninae] and also Vulcanoctopus, Grimpella, Japetella, Vitreledonella and 2 argonautoid representatives, Argonauta and Tremoctopus). The authors used both Bayesian and maximum likelihood methods to analyse mitochondrial and nuclear sequence data separately as well as concatenating all sequence data into a single dataset for analysis. They analysed the data coded as DNA and also as amino acids. The following diagram is a strict consensus tree, depicting phylogenetic relationships supported by all analyses and a proposed classification derived from the analysis:

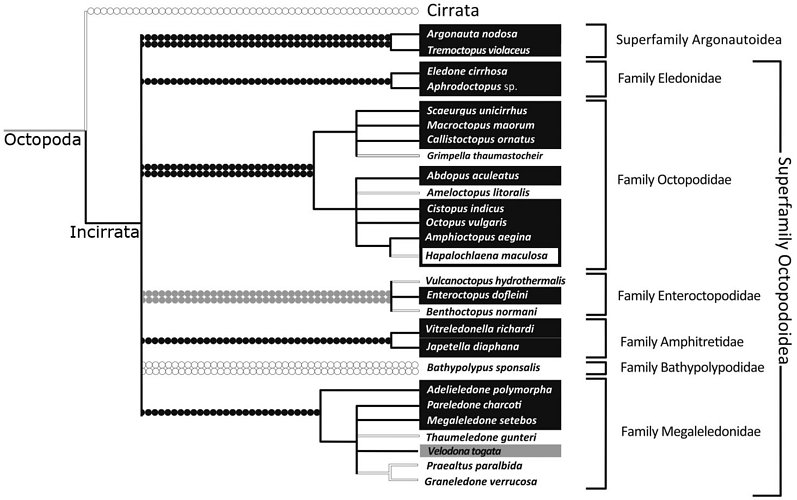
Figure. Phylogenetic tree and classification from Strugnell et al., (2013, slightly modified).
Based on this analysis, both characters show multiple derivation/losses. Sucker seriation appears to be a more conservative character than the presence/absence of an ink sac and appears to be fixed at family level. The presence/absence of an ink sac is a much more volatile character and it has been lost in numerous lineages. It is clear that previously proposed subfamilies of the Octopodidae (recognised in earlier works) that are based on these characters do not reflect evolutionary history.
*Department of Genetics, La Trobe Institute for Molecular Science, La Trobe University, Bundoora 3086, Australia
Life history
Where known, incirrate octopods have a life span of 6 months to 4 years; one year is typical. Those living to 4 years are large and/or cold water species. Most species appear to be terminal spawners, i. e. they lay all eggs toward the end of their life-span and die shortly after the last of the brooded embryos hatch. Some species, however, lay several egg batches and feed between spawning periods. Spawning in the epipelagic incirrates is in this latter mode but egg batches overlap (i.e. a second batch is deposited before the first one hatches).
All incirrate octopods, benthic and pelagic, brood their eggs until the young hatch. Egg strings are mostly fixed to a substrate although some species carry their eggs on the arms. Specialized brooding habits are present in pelagic incirrates. In Argonauta the dorsal arms secrete a "shell" that functions as a brood chamber and in Tremoctopus and the bolitaenids the arms form brood-chambers. In Ocythoe, the eggs develop in the oviducts and in Vitreledonella, apparently, in the mantle cavity.
References
Carlini, D. B. 1998. The phylogeny of coleoid cephalopods inferred from molecular evolutionary analyses of the cytochrome oxidase I, muscle actin, and cytoplasmic actin genes. Ph.D. diss. Coll. William and Mary, 273 pp.
Carlini, D. B. and J. E. Graves. 1999. Phylogenetic analysis of cytochrome c oxidase I sequences to determine higher-level relationships within the coleoid cephalopods. Bull. Mar. Sci., 64: 57-76.
Hochberg, F. G., M. Nixon and R. B. Toll. 1992. Order Octopoda Leach, 1818. In: Sweeney, M. J., C. F. E. Roper, K. M. Mangold, M. R. Clarke and S. v. Boletzky (eds.) "Larval" and juvenile cephalopods: A manual for their identification. Smithson. Contr. Zool., 513:1-282.
Mangold, K. 1989. Cephalopodes. Traité de Zoologie. Tome V. Masson, Paris. 804pp.
Naef, A. 1921/23. Cephalopoda. Fauna und Flora des Golfes von Neapel. Monograph, no. 35.
Robson, G. C. (1932). A monograph of the Recent Cephalopoda. Part II. The Octopoda (excluding the Octopodinae). Brit. Mus. (Nat. Hist.), London.
Strugnell, J. M., M. D. Norman, M. Vecchione, M. Guzik and A. L. Allcock. 2013. The ink sac clouds octopod evolutionary history. Hydrobiologia (online, 28 May), 21 pp.
Taki, I. 1962. On species newly added to the fauna of Japanese Cephalopoda. Zool. Mag., Tokyo, 71: 397-398. [In Japanese.]
Taki, I. 1964. On eleven new species of the Cephalopoda from Japan, including two new genera of Octopodidinae. Jour. Fac. Fish. Animal Husb., Hiroshima Univ., 5: 277-343.
Thore, S. 1949. Investigations on the "Dana" Octopoda. Dana-Report No. 33, 85pp.
Voight, J. R. 1997 -- Cladistic analysis of the octopods based on anatomical characters. J. Moll. Stud. 63: 311-325.
Young, J. Z. 1971. The Anatomy of the Nervous System of Octopus vulgaris. Claredon Press, Oxford.
Young, R. E., M. Vecchione and D. Donovan. 1999. The evolution of coleoid cephalopods and their present biodiversity and ecology. South African Jour. Mar. Sci. .
About This Page
Katharina M. Mangold (1922-2003)

Laboratoire Arago, Banyuls-Sur-Mer, France

University of Hawaii, Honolulu, HI, USA
Page copyright © 2013 Katharina M. Mangold (1922-2003) and
 Page: Tree of Life
Incirrata . Common octopods, octopuses or devilfishes.
Authored by
Katharina M. Mangold (1922-2003) and Richard E. Young.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Incirrata . Common octopods, octopuses or devilfishes.
Authored by
Katharina M. Mangold (1922-2003) and Richard E. Young.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- Content changed 07 July 2013
Citing this page:
Mangold (1922-2003), Katharina M. and Richard E. Young. 2013. Incirrata . Common octopods, octopuses or devilfishes. Version 07 July 2013 (under construction). http://tolweb.org/Incirrata/20087/2013.07.07 in The Tree of Life Web Project, http://tolweb.org/





 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site