Odonata
Dragonflies and damselflies
John W. H. Trueman and Richard J. Rowe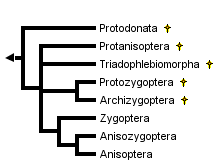

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
Odonata are an order of aquatic palaeopterous insects. There are about 6500 extant species in just over 600 genera. Adult odonates are medium to large in size, often conspicuous and/or brightly colored insects and are aerial predators hunting by sight. They generally are found at or near fresh water although some species roam widely and may be found far from their breeding sites. The larvae are predatory, aquatic and occur in all manner of inland waters.
In some countries, notably Japan, Odonata have long been a popular subject of art and culture, and rank with butterflies and birds as a topic of popular scientific interest. In the European folk tradition, odonates are generally accorded a less favourable status as "horse-stingers" or "devil's darning needles". In fact they neither sting nor bite and all species are completely harmless. If anything, Odonata are beneficial to humans because as voracious aquatic predators they assist in the control of insect pests.
To some extent the presence of odonates may be taken as an indicator of ecosystem quality. Local faunal composition may be strongly affected by any change in water flow, turbidity, etc., or in aquatic or waterside vegetation. The greatest numbers of species are found at sites which offer a wide variety of microhabitats.
Inland fishermen may know dragonfly larvae as "mud-eyes" and use them as bait. Adult dragonflies are a minor food item in some countries, and the larvae sometimes have been used to control pest insects (eg, mosquitos in domestic water tanks). But, for the most part, Odonata are of little economic importance. Their main attraction for humans is aesthetic.
The modern order is divided into two main suborders: Zygoptera (damselflies) and Anisoptera (dragonflies). The common name "dragonfly" also is used for the whole order. More than one-half of all species are tropical but odonates of both major suborders occur in every faunal region except Antarctica. A third suborder, Anisozygoptera, largely known from fossils, is represented by one extant species in Japan and one in the Himalayas.


Sympetrum corruptum. USA. Anisoptera; family Libellulidae.
Copyright © 1997 Larry Jon Friesen.


Epiophlebia superstes. Japan. Anisozygoptera; family Epiophlebiidae, emergent adult on its larval skin. Photo: Takashi Aoki
Copyright © 1997 Takashi Aoki.
Superfamily and family distributions in Odonata reflect ancient vicariance events such as the break-up of the southern continent Gondwana. Some genera and species are widespread. Others are highly local in distribution. Some families are restricted to cool streams or rivers, others to ponds or other still waters, and some to marshy places. The most species-rich and widespread family is the mainly tropical Libellulidae (Anisoptera). The most restricted is the monotypic Hemiphlebiidae (Zygoptera), only known from six or so small reedy pools in south-eastern Australia.
The fossil record for most modern families dates back to the Jurassic or else the Cretaceous period. Fossil species not assignable to any of the the three extant suborders are placed conventionally into one of four fossil suborders: Protozygoptera, Archizygoptera, Protanisoptera and Triadophlebiomorpha. A separate order, Protodonata, occasionally regarded as a suborder of Odonata, contains some very large to enormous Upper Carboniferous and Permian fossil odonatoid species. The largest of these "giant dragonflies", Meganeuropsis permiana, measured about 720 mm from wingtip to wingtip. The wingspan of modern anisopterans ranges from less than 20 mm (eg, Nannodiplax rubra, Libellulidae) to more than 160 mm (eg, Petalura ingentissima, Petaluridae): those of modern Zygoptera range from about 18 mm (eg, Agriocnemis pygmaea, Coenagrionidae) to about 190 mm (Megaloprepus caerulatus, Pseudostigmatidae).
Characteristics: adults
General: The head is large, concave behind, on a flexible neck. The compound eyes are large and either widely separated (all Zygoptera, some Anisoptera), just touching, or extensively fused along the mid-line. The face carries three ocelli and a pair of short, bristle-like antennae. The prothorax is small, the mesothorax and metathorax are large and fused into a single, strong pterothorax. The legs are weak, suited to perching and to holding prey but not to walking. The abdomen is long, rarely any shorter than the length of one wing, and flexible, with 10 visible segments, and terminates in clasping organs in both sexes. In Zygoptera the abdomen almost always is thin and cylindrical. Females of all zygopteran and several anisopteran families carry a prominent ovipositor under abdominal segments 9-10. Males always possess secondary genitalia on the underside of abdominal segments 2-3.
Resting attitude: The wing veins of Odonata are fused at their bases and the wings cannot be folded over the body at rest. Almost all Anisoptera settle with the wings held out sideways or slightly downward. Most Zygoptera settle with the wings held together, dorsal surfaces apposed. However, the zygopteran thorax is so oblique that when held in this way the wings fit neatly along the top of the abdomen. They do not appear to be held straight 'up' as in butterflies or mayflies. In a few zygopteran families the wings are held horizontally at rest, and in one anisopteran genus (Cordulephya, Corduliidae) the wings are held in the typical damselfly resting position.
Wing venation: Adult Odonata possess two pairs of equal or subequal wings. There appear to be only five main vein stems. A Nodus is formed where the second main vein (subcosta) meets the leading edge of the wing. In most families a conspicuous pterostigma is carried near the wing tip.
Identification as Odonata can be based on the venation. The only likely confusion is with some lacewings (order Neuroptera) which have many crossveins in the wings. Until the early years of the 20th century Odonata were often regarded as being related to lacewings and were given the ordinal name Paraneuroptera, but any resemblance between these two orders is entirely superficial.
In Anisoptera the hindwing is broader than the forewing and in both wings a crossvein divides the discoidal cell into a Triangle and Supertriangle.


Wings of Archipetalia auriculata (Neopetaliidae) *taxonomic note
Photo: J. W. H. Trueman,
Copyright © 1997 J. W. H. Trueman
Rarely, the crossvein runs obliquely across the cell, causing either the Triangle or Supertriangle to appear four-sided.
In Zygoptera the two pairs of wings are almost exactly equal in shape, size and venation. There may be very numerous crossveins:


Hindwing venation of Matrona japonica (Calopterygidae).
or rather few:


Hindwing of Austroargiolestes icteromelas (Megapodagrionidae).
The venation in Anisozygoptera is intermediate between that of the two major suborders:

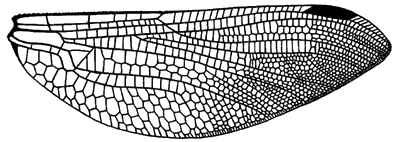
Hindwing venation of Jurassic fossil species Heterophlebia buckmani.
Characteristics: larvae
The most obvious characteristic shared by all odonate larvae is a conspicuous grasping labium (lower lip: mask), used for capturing prey.


At rest the mask is held folded underneath head and thorax, extending back as far or further than the front legs and in some families far enough forward to cover the face below the compound eyes. In prey capture the labium is shot rapidly forward and prey is grasped with paired, hand-like palps.
Even from above and with the mask retracted, identification of larvae to order and suborder is very easy, based on several other features.
Life Cycle and Behavior
Odonate larvae are non-discriminate hunters which will eat any animal as large as or smaller than themselves, including their own species. Small vertebrates such as tadpoles and fish fry are not immune from attack. Prey may be stalked or ambushed. Captured prey is pulled back using powerful muscles in the labium and chewed by strong mandibles. More details on labium structure and larval predatory activity are available.
Almost all odonate larvae are aquatic. They occur in every sort of water body from soaks and seepages to streams and rivers, lakes, temporary pools and water-filled tree holes. A few species tolerate moderate salinity, a few others have semi-terrestrial larvae which roam across the surface of bogs and swamps at night. A half-dozen or so fully terrestrial larvae are known from distantly related families. These animals live in leaf litter layers in tropical rainforests.
As they grow, larvae undergo approximately 10-20 molts, over a time between 3 months and about 6-10 years depending on species. Instar number is not always fixed but may depend on seasonal conditions and food supply. Wing pads develop externally from about the 6-7th instar. Metamorphosis is direct without a pupal stage and emergence takes place on a fixed support out of the water, sometimes a considerable distance from the water's edge. The newly emerged adult flies away from water for a few days to feed and mature, during which time the full adult color develops. Teneral (new) adults can be recognised by a glassy sheen of the wings. Additional color changes occur later in life in some species.
Adult Odonata are visually oriented hunters with exceptional aerobatic ability and extremely acute eyesight. Many are strong fliers, and to catch them can be extremely difficult. Males tend to congregate around the breeding sites where they may be seen either perched on waterside vegetation, hovering over small territories or hawking up and down in search of females. Females of many species spend much time away from the water, only appearing to mate and lay eggs, but some congregate with the males.
Most adults are long-lived. In cold climates some over-winter in sheltered places and in the dry tropics some aestivate through the dry season. Some undertake long dispersal flights, including transoceanic journeys, but others remain tightly associated with their larval habitat.
Mating: Odonate mating is an elaborate process. The male clasps the female by the head (Anisoptera) or prothorax (Zygoptera). The pair then fly together in tandem (male in front, female behind), often to a perch. The female bends her abdomen forward and downward to form the "wheel" position and connect with the secondary genitalia on segments 2-3 of the male, which previously have been charged with sperm from the primary genital opening on segment 9. Complex sperm displacement and sperm transfer activities then occur. A mating may last from several seconds to several hours, according to species.
In some species the pair lay eggs together, maintaining the tandem hold. In others the male hovers above the female while she lays her eggs. In some the male returns to his territory or perch while the female oviposits alone.


Xanthocnemis zealandica ovipositing (New Zealand)
Photo: R.J. Rowe. Copyright © 1997 R.J. Rowe
Eggs are laid into plant tissues on, above, or below the water surface, or, in species without a functional ovipositor, are deposited onto the water surface or inserted into sand or mud. Egg development mostly is direct (ie, without diapause) and eggs takefrom 7-9 days to several months to hatch, according to species. Egg-stage overwintering is common in temperate climates. The egg hatches as a "prolarva" or 1st instar (actually a pharate 2nd instar) which wriggles to water if not already in it and then immediately undergoes ecdysis to the 2nd instar (first feeding, first free-living) stage.
Discussion of Phylogenetic Relationships
Higher Relationships
The relationships of Odonatoidea (= Odonata plus Protodonata) to the other pterygote insects continues to be much debated. For an outline see the attached note on Pterygote higher relationships.
Subordinal relationships in Odonata
Within the order Odonata, debate rages on several fronts. Which suborders are monophyletic and which are paraphyletic? Which fossil groups are "ancestral to" or closely related to which modern suborders? Is Zygoptera a paraphyletic basal grade from which Anisozygoptera and Anisoptera arose or are Zygoptera and Anisoptera each monophyletic groups, and is each derived from within suborder Anisozygoptera? There appears to be general agreement only that
- Odonata is a monophyletic group which has been separated from Ephemeroptera, Neoptera, and the extinct Palaeodictyopteroidea since at least the lowermost Upper Carboniferous
- The three extant suborders are closely related
- Fossil suborder Archizygoptera is a subset of fossil suborder Protozygoptera
- Modern Anisoptera is monophyletic
Wing venation
Hypotheses of the major phylogenetic relationships within Odonata depend critically on interpretation of the wing venation. Different schemes of wing vein homology across the suborders and alternative hypotheses of character-state transformation or polarity lead directly to different evolutionary hypotheses. Despite many efforts, consensus remains elusive. Attached is a brief note about differing views on Odonate wing venation.
Subordinal relationships
Consistent with their various conclusions on the evolution of the wing, different authors have proposed different subordinal phylogenies.
Handlirsch (1906-08) regarded Anisozygoptera as a "stem group" from which Anisoptera and Zygoptera arose as separate lineages. Tillyard (1928) regarded Zygoptera as basal, with Anisozygoptera derived from within Zygoptera and Anisoptera derived from within Anisozygoptera. Tillyard and Fraser (1938-40) treated Zygoptera and Anisozygoptera + Anisoptera as non-sister monophyletic groups, the former derived from Protozygoptera and the latter from Protanisoptera, but Fraser (1957) reverted to the Tillyard (1928) arrangement.
Hennig (1981) made Zygoptera the monophyletic sister group to Anisozygoptera + Anisoptera. He placed Anisozygoptera sister to Anisoptera. Carle (1982) followed Handlirsch (1906-08) in treating Anisozygoptera as basal but regarded both Zygoptera and Anisoptera as monophyletic. Carle and Wighton (1990) derived a polyphyletic Zygoptera by convergent reduction in the wing veins from anisopteran ancestors.
Trueman (1996) applied cladistic parsimony analysis to a set of wing vein morphological characters, finding in favor of Fraser's (1957) "paraphyletic Zygoptera, paraphyletic Anisozygoptera" hypothesis. In contrast, Bechly (1995, 1998), using a non-quantitative approach (Hennig's argumentation scheme) that assumes prior knowledge of ancestral character states and involves ad-hoc resolution of character conflict, favored a monophyletic Protozygoptera that is sister to the modern suborders, a paraphyletic Anisozygoptera, a monophyletic Zygoptera and monophyletic Anisoptera. Bechly has more recently withdrawn his system. Nel et al. (1993) and Lohmann (1996) made similar assumptions to Bechly and came to roughly similar conclusions. Lohmann established a strong case for regarding extant Epiophlebiidae as the sister group to the rest of the Anisoptera, rather than as Anisozygoptera. It would thus be logical to extend the Anisoptera to incorporate the Epiophlebiidae.
Misof et al (2001) estimated the relationships of Anisoptera based on molecular sequence data, an approach that holds great promise for resolving the major, longstanding questions in odonate phylogeny. Their results differed significantly from those of Bechly (1998). Unfortunately, the terminal branches on their gene tree were in general long while internal branches were exceedingly short, a situation that makes the resolution of phylogenetic relationships exceedingly difficult. Misof et al. succeeded in recovering the majority of recognised family groupings in Anisoptera but had limited success with the inter-family relationships. On the basis of current fragmentary reports of data from other genes and other odonate taxa it seems likely that this hard-to-resolve pattern of long and short branches with marked non-stationarity in key parameters will hold across the whole order.
Rehn (2003) has produced a synoptic overview of odonate phylogeny that deserves to be considered in some detail. Rehn's scheme produces patterns with much appeal in both the Zygoptera (considered monophyletic) and in the Anisoptera + Epiophlebia (termed the 'Epiprocta'). The monophyly of the Zygoptera is supported by six characters, the two most important being the transverse head structure and the widely separated eyes, considered as derived characters. We are as yet unconvinced of the character polarity assumed here.
Kjer (2004) used the 18S sequence to evaluate relations in the Insecta. While generally very satisfactory, within the Odonata this study led to a rather unusual tree, with Epiophlebia at the base, the Anisoptera as a transition series, and with the Zygoptera as sister to a branch of the Libellulidae. Kjer comments on the credibility of the general tree produced and on the peculiar difficulties with the 'structure' within the Odonata.
Both Saux et al (2003) and Hasegawa and Kasuya (2006) conducted limited DNA sequence studies directed at determining the basal structure of the odonate tree. Both these studies recovered a paraphyletic Zygoptera and a monophyletic Anisoptera. Other broader, but less focussed, studies have recovered monophyletic Zygoptera and monophyletic Anisoptera. Trueman (2007) has reviewed the history of phylogenetic research in Odonata and points to the effects of vein nomenclature systems in influencing tree structures where wing characters are scored.
Thus, at the present time, the jury remains firmly out. Even so, convergence toward one of two rival phylogenies can be discerned. The pattern of subordinal relationships would appear to be either as Tillyard (1928) and Fraser (1957) have it:
============================= Protodonata
|
| ========================== Protanisoptera
=====| |
| | ======================= Protozygoptera
===| |
===| ============ Zygoptera (Coenagrionoidea)
| |
=Zygoptera=| ========= Zygoptera (Lestoidea, Calopterygoidea)
| |
===| ====== Anisozygoptera (eg, Epiophlebia)
===|
| === Anisozygoptera (eg, Heterophlebia)
===|
=== Anisoptera
or else something along the lines first proposed by Handlirsch (1906-08). Using conventional names this may be represented as:
================================== Protodonata
|
| =============================== Protanisoptera
=====| |
| | ============================ Protozygoptera
===| |
===| |======== Zygoptera
| |
==Anisozygoptera===| ====== Anisozygoptera (eg, Epiophlebia)
| |
===| === Anisozygoptera (eg, Heterophlebia)
===|
=== Anisoptera
. . . although within each broad class of phylogenies both the number and the ordering of branches within Zygoptera and Anisozygoptera are much disputed.
Classification
The established and widely accepted classification of extant families is as follows:
Zygoptera
- Hemiphlebioidea
- Hemiphlebiidae
- Coenagrionoidea
- Coenagrionidae
- Isostictidae
- Platycnemididae
- Platystictidae
- Protoneuridae
- Pseudostigmatidae
- Lestoidea
- Lestidae
- Lestoideidae
- Megapodagrionidae
- Perilestidae
- Pseudolestidae
- Synlestidae
- Calopterygoidea
- Amphyipterygidae
- Calopterygidae
- Chlorocyphidae
- Dicteriastidae
- Euphaeidae
- Polythoridae
Anisozygoptera
- Epiophlebiidae
Anisoptera
- Aeshnoidea
- Aeshnidae
- Gomphidae
- Neopetaliidae
- Petaluridae
- Cordulegastroidea
- Cordulegastridae
- Libelluloidea
- Corduliidae
- Libellulidae
(Classification from Watson and O'Farrell, 1991)
Alternative classification schemes have been recently proposed which erect many new taxonomic divisions and coin new names for old categories. To maintain stability we recommend use of the established and generally accepted classification until such time as the superiority of one or another of the newly proposed forms can be clearly established.
A listing of accepted extant species and of synonyms, maintained by Martin Schorr, Martin Lindeboom, and Dennis Paulson, is hosted at the University of Puget Sound. The family structure used in this listing varies slightly from that advocated above.
References
Bechly, G. H. P., 1995. Morphologische Untersuchungen an Fluegelgeaeder der rezenten Libellen und deren Stammgruppenvertreter (insecta; Pterygota; Odonata) unter besonderer Beruecksichtigung der phylogenetischen Systematik und des Grundplanes der Odonata. Petalura (Spec. Vol.) 1: 1-341.
Bechly, G. 1998. The Phylogenetic Systematics of Odonata. Internet website http://www.bernstein.naturkundemuseum-bw.de/odonata/phylosys.htm developed and maintained by the author - updated 2008.
Carle, F. L., 1982. The wing vein homologies and phylogeny of the Odonata: a continuing debate. Soc. int. Odonatol. rapid Comm. 4, x,66 pp.
Carle, F. L., and D. C. Wighton, 1990. Insects from the Santana formation, Lower Cretaceous, of Brazil. 3. Odonata. Bulletin American Museum of Natural History 195: 51-68.
Fraser, F. C., 1957. A reclassification of the order Odonata. Sydney. R. Zool. Soc. NSW.
Handlirsch, A., 1906-08. Die fossilen Insekten und die Phylogenie der rezenten Formen. Ein Handbuch fur Palaontologen und Zoologen. Leipzig. Engelmann.
Hasegawa, E. and Kasuya, E. 2006. Phylogenetic analysis of the insect order Odonata using 28S and 16S rDNA sequences: a comparison between datasets with different evolutionary rates. Entomological Science 9: 55-66.
Hennig, W., 1981. Insect Phylogeny. New York. Wiley.
Kjer, K. M. 2004. Aligned 18S and insect phylogeny. Systematic Biology 53: 506-514.
Lohmann, H., 1996. Das phylogenetische System der Anisoptera (Odonata). Entomol. Z. 106(6):209-252, 106(7):253-296, 106(9):360-367.
Misof, B., and G. Fleck. 2003. Comparative analysis of mt LSU rRNA secondary structures of Odonates: structural variability and phylogenetic signal. Insect Molecular Biology 12(6): 535-547.
Misof, B., A. M. Rickert, T. R. Buckley, G. Fleck, and K. P. Sauer, 2001. Phylogenetic signal and its decay in mitochondrial SSU and LSU rRNA gene fragments of Anisoptera. Molecular Biology and Evolution 18(1):27-37.
Nel, A., X. Martinez-Delclos, J.-C. Paicheler, & M. Henrotay, 1993. Les "Anisozygoptera" fossiles: phylogeneie et classification (Odonata). Martinia (hors ser.) 3: 1-311.
Rehn, A. C. 2003. Phylogenetic analysis of higher-level relationships of Odonata. Systematic Entomology 28(2): 181-239.
Saux, C., C. Simon, and G. S. Spicer. 2003. Phylogeny of the dragonfly and damselfly order Odonata as inferred by mitochondrial 12S ribosomal RNA sequences. Annals of the Entomological Society of America 96(6):693-699.
Tillyard, R. J., 1928. The evolution of the order Odonata. Part 1. Introduction and early history of the order. Records of the Indian Museum 30: 151-172.
Tillyard, R. J., and F. C. Fraser, 1938-40. A reclassification of the order Odonata. Austr. Zool. 9: 125-169, 195-221, 359-390.
Trueman, J. W. H., 1996 [1991]. A preliminary cladistic analysis of odonate wing venation. Odonatologica 25: 59-72.
Trueman, J. W. H. 2007. A brief history of the classification and nomenclature of Odonata. Zootaxa 1668:381-394.
Watson, J. A. L., and A. F. O'Farrell, 1991. Odonata (Dragonflies and Damselflies). Ch. 17 in CSIRO (ed.) The Insects of Australia. A textbook for students and research workers. 560 , 600 pp. 2 volumes. Carlton. Melbourne University Press. pp. 294-310.
General accounts of biology
Corbet, P. S., 1999. Dragonflies: Behavior and Ecology of Odonata. Cornell University Press, Ithaca, NY. and Harley Books, Colchester, UK. revised edition 2004
Information on the Internet
World lists of dragonfly species:
- The Schorr, Lindeboom and Paulson listing
- The van Tol/Species2000 listing
- The Dragonflies species information plus literature (species listing from van Tol)
A growing number of organizations and dragonfly enthusiasts maintain websites about dragonflies. Substantial and notable sites, as of October, 2009, include the following:
International Organizations:
- WDA Worldwide Dragonfly Association. Publishers of the journal INTERNATIONAL JOURNAL OF ODONATOLOGY.
- FSIO Foundation SIO. Publishers of the journal ODONATOLOGICA.
Regional Organisations:
- IORI The International Odonata Research Institute. This organization, based in Florida, USA, is a good source of general information on Odonata and maintains extensive links to other dragonfly web pages.
- DSA The Dragonfly Society of the Americas brings together dragonfly workers and enthusiasts in North and South America.
Other notable Odonata pages:
- Takashi Aoki (Japan)
- Roy Beckemeyer (Kansas, USA)
- Martin Peterson (Sweden)
- Gordon Ramel (UK)
- Richard Rowe (Australia)
- Slater Museum page Denis Paulson's site
- Digital Dragonflies A page with some high-quality images of Odonata.
- Phylogeny (Gunter Bechly) One view of odonate systematics using Hennig's argumentation scheme - now of historical interest.
- Odonata Links on the World Wide Web. Oregon Dragonfly and Damselfly Survey.
Title Illustrations

| Scientific Name | Argia |
|---|---|
| Location | North America |
| Copyright |
© 1997 Joseph L. Spencer

|
| Scientific Name | Sympetrum sanguineum |
|---|---|
| Location | Romania |
| Specimen Condition | Live Specimen |
| Identified By | Horia Bogdan |
| Sex | Female |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 2.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 2.0.
|
| Copyright |
© Horia Bogdan

|
About This Page
Special thanks to Takashi Aoki for permission to use the image of Epiophlebia superstes, and to Larry J. Friesen, Peter Marsack, Stephen Richards and Joseph L. Spencer for other images.
Line drawings copyright © 1997, John W. H. Trueman and Richard J. Rowe.
This page compiled by:
John W. H. Trueman

Australian National University, Canberra, Australia
Richard J. Rowe

James Cook University of North Queensland, Townsville, Queensland, Australia
Correspondence regarding this page should be directed to John W. H. Trueman at
Page copyright © 2009 John Trueman & Richard Rowe
All Rights Reserved.
- Content changed 16 October 2009
Citing this page:
Trueman, John W. H. and Richard J. Rowe. 2009. Odonata. Dragonflies and damselflies. Version 16 October 2009. http://tolweb.org/Odonata/8266/2009.10.16 in The Tree of Life Web Project, http://tolweb.org/










 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site